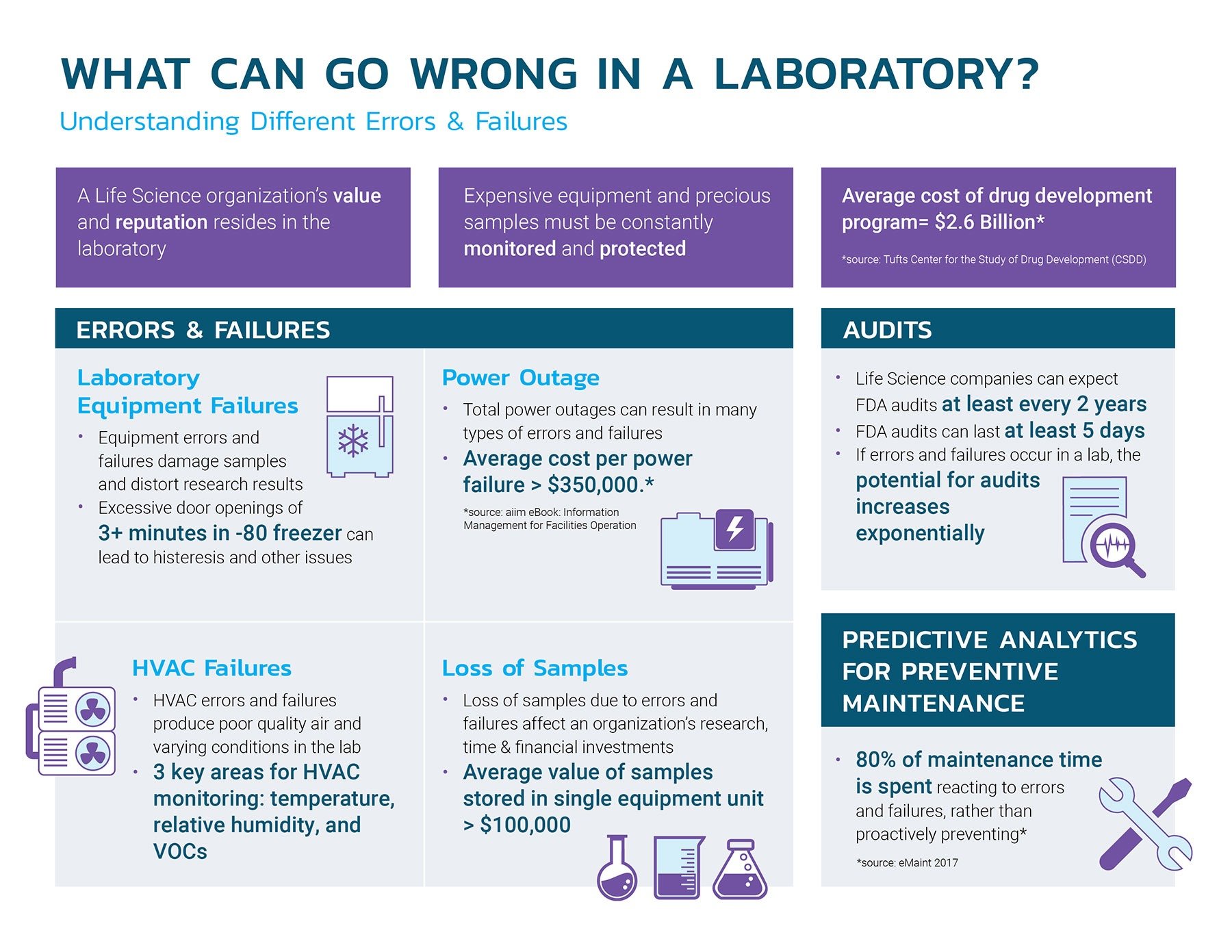
What Can Go Wrong in a Laboratory if Not Using Monitoring?
Understanding Different Errors and Failures in a Laboratory Environment
A life science organization’s value and reputation reside in the laboratory. With the average cost of a drug development program being more than $2.6 billion, having proper safeguards and reporting systems compliant with the highest US and International standards are key to preventing sample loss and delays.